In 1906, German doctor Alois Alzheimer detected plaques in the brain of a patient with dementia. It was nearly 80 years later that molecular biologists learned the plaques were made up of a pathological form of a protein called amyloid.
“I remember that discovery,” says biochemist Ulf Neumann, who has been working on one of two experimental treatments for Alzheimer’s disease at Novartis in the Neuroscience group since 2001. “Everybody thought, now that we know what it is, we can do something about it.”
Effective therapies, however, have been elusive. Since the discovery of amyloid, scientists have had to continually refine their ideas about the cause of Alzheimer’s disease. A prevailing theory, called the amyloid cascade hypothesis, details a complex sequence of steps that starts with dysregulation of the normal amyloid processing pathway. This leads to soluble and then insoluble forms of amyloid, which form plaques that eventually cause neurodegeneration and memory and thinking problems. After a certain point of progression, the cascade may be irreversible and trigger other pathological processes, so researchers at Novartis and elsewhere have developed a new therapeutic strategy: stop Alzheimer’s disease before it starts. Or at least before it progresses to clinical symptoms.
The amyloid cascade begins when toxic forms of amyloid begin to accumulate in the brain and clump to form sticky plaques. Research suggests that amyloid buildup progresses slowly, over a span of decades.
“Five to ten years prior to diagnosis, a person may already have a good amount of amyloid in the brain, but they might not forget things or have any problems with daily life,” says Neumann.
Some scientists question the amyloid cascade hypothesis because of this disconnect between the buildup of amyloid and the beginning of symptoms. Further, at later stages in the disease, other non-amyloid brain changes occur that appear along with symptoms. Since amyloid buildup does not correlate well with symptoms, perhaps amyloid is not the best target for interventions, they reason.
But researchers counter this argument with an alternate proposal: Alzheimer’s may result from a series of events that trigger one another like falling dominoes. Amyloid is the first visible change in the brain, says Neumann. The next is the buildup of the protein tau, in what are known as tau tangles. Tau tangles always appear after amyloid plaques, though no clear mechanism has been found to link them. Once tau tangles appear, Alzheimer’s symptoms are likely to follow.
“We think that amyloid is probably most important very early in the disease as an initiator of other detrimental biological processes,” says J. Michael Ryan, Neurodegeneration Therapeutic Area Head at Novartis Pharmaceuticals in New Jersey. “So we’d like to intervene with anti-amyloid therapy at the earliest feasible time.”
An upcoming Alzheimer’s disease prevention trial is designed to test early intervention in a genetically defined high-risk population, before symptoms begin. The trial, developed collaboratively by Novartis and the Banner Alzheimer’s Institute, will begin in late 2015 and will test two experimental drugs from Novartis that target the early stages of the amyloid cascade.
“We are very invested in the hypothesis that treating earlier is more likely to be beneficial,” says Pierre N. Tariot, Director of the Banner Alzheimer’s Institute and a Professor at the University of Arizona College of Medicine.
One of the Novartis experimental agents is designed to prevent the formation of amyloid in the first place. The other is made to coax the body’s immune system to clear toxic amyloid. While both drugs target the amyloid cascade, it isn’t yet known if they will prevent or delay Alzheimer’s disease because the amyloid cascade hypothesis is still just that, a hypothesis in need of further testing.
The prevention trial could lend credence to the amyloid cascade hypothesis by determining if there is a link between early reductions in amyloid and delayed Alzheimer’s onset. To make this link, the trial will employ biomarkers that gauge reductions in amyloid levels in response to the experimental therapies, such as measures of amyloid levels in cerebral spinal fluid and brain scans that track the burden of amyloid plaques. The trial will also carefully measure the earliest evidence of cognitive impairment.
Evidence of a connection between reduced amyloid and delayed symptoms could pave the way for accelerating future trials. For instance, trials could look at early biomarker changes in response to an experimental agent instead of waiting years for symptoms to arise in volunteers.
“This trial could teach us how to use biomarkers as substitutes for clinical outcomes in future studies,” says Tariot. “If it does, we can start doing shorter, more efficient biomarker-based studies so we can make faster go/no go decisions about new Alzheimer’s drugs.”
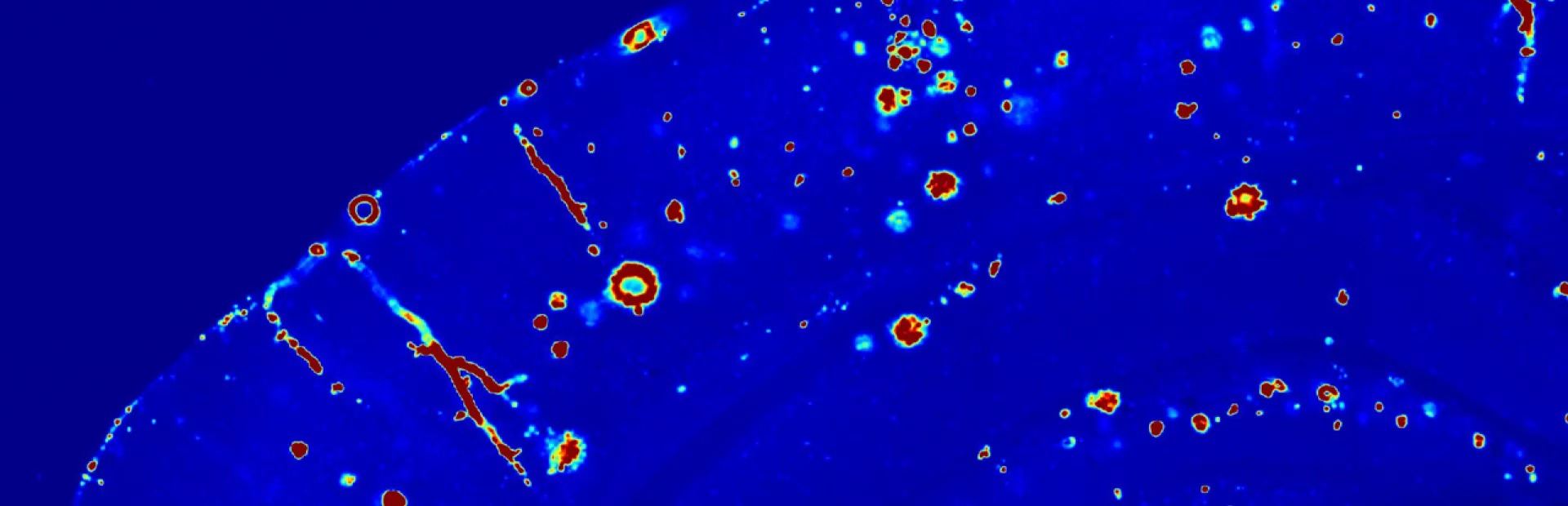
Main image: Beta-amyloid protein (in red) is beginning to accumulate in this brain tissue. Researchers suspect that it plays a role in the onset of Alzheimer’s disease. Image: Nikita Rudinskiy/Novartis
Experimental treatments for Alzheimer’s disease put the amyloid cascade hypothesis to the test.