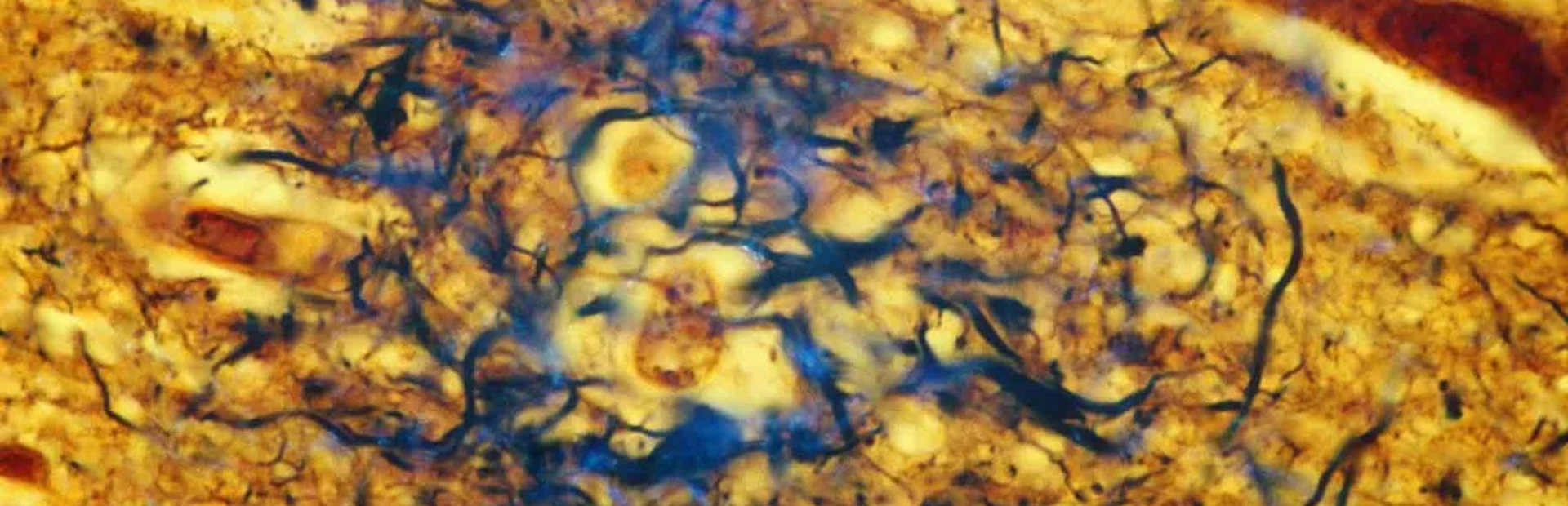
Jamie Tyrone got her first look at Alzheimer’s disease at the age of 10, in the mid-1970s, during a visit with her great-grandmother. “She was lifeless,” Tyrone recalls. “Her eyes were empty.”
Nearly four decades later, Tyrone signed up for an online research study to learn about her genetic risk for 23 diseases. Alzheimer’s disease didn’t even cross her mind. When she received results pointing to a 91% lifetime risk of Alzheimer’s disease, she was devastated. But what shook Tyrone most deeply was that, despite this knowledge, she was powerless. There are no proven ways to prevent Alzheimer’s disease.
Now, however, a new clinical trial is in the works that will test two experimental treatments from Novartis in individuals who have an elevated risk of developing Alzheimer’s disease due to their genetic make-up and age. Although Tyrone is too young to participate in the trial, she plans to follow its progress. Preliminary research suggests that these treatments may prevent or delay the onset of symptoms. The trial—which was jointly designed by teams at Novartis and the Banner Alzheimer’s Institute in Phoenix, Arizona—is unusual because of its focus on preventing the disease in older adults.
To be at this stage now where we can design a prevention trial that matches people with genetic risk to innovative therapies is a dream come true.
J. Michael Ryan, Novartis Pharmaceuticals Neurodegeneration Therapeutic Area Head
“To be at this stage now where we can design a prevention trial that matches people with genetic risk to innovative therapies is a dream come true,” says J. Michael Ryan, who is the Novartis Pharmaceuticals Neurodegeneration Therapeutic Area Head overseeing efforts to develop new Alzheimer’s treatments at the company.

If successful, the trial could bring hope to Tyrone and others like her. Tyrone tested positive for two copies of a gene variant called APOE4, which is associated with an elevated risk of late-onset Alzheimer’s disease. Tyrone, whose father died with Alzheimer’s disease three years ago, got a copy from each parent. She has the same genetic profile as patients who will be enrolled into the Novartis-Banner trial. If preventive therapies prove effective, they would give Tyrone a way to take action to lower her Alzheimer’s disease risk, similar to the way people use statins and aspirin to lower their heart disease risk.