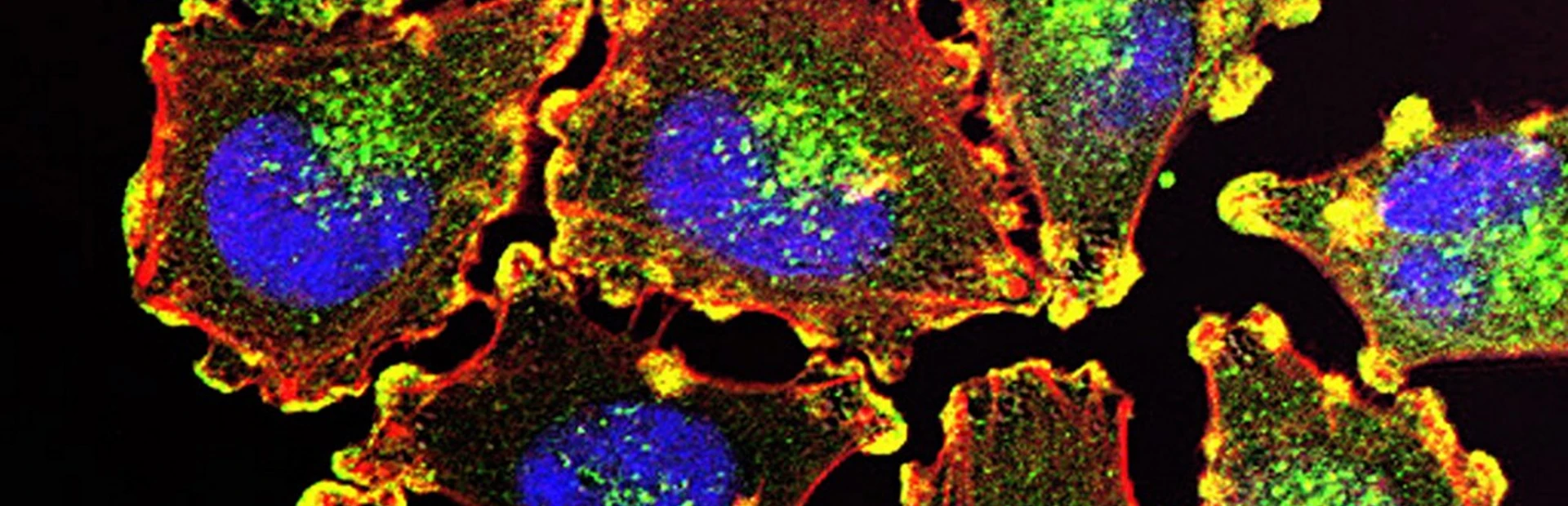
Cancer cells all contain dense networks of interacting genes. Some genes cancer cells can do without. Others drive the biology that causes them to grow uncontrollably.
Because the drivers have functional importance in cancer, they represent potential drug targets. Now Novartis scientists from across the globe have reported major progress toward finding them.
On July 27, the team released results from Project DRIVE – a massive screening effort carried out by more than 100 researchers to expose the genes that cancer cells depend on for their growth and survival.
“With this robust and comprehensive screening campaign, we’ve developed an encyclopedia of the functional drivers in cancer,” says Tobias Schmelzle, Director of Target Identification and Validation in Oncology at the Novartis Institutes for BioMedical Research (NIBR) and a co-corresponding author of the Project DRIVE paper, which was published in Cell. Schmelzle helped lead the screening effort and participated in the downstream data analysis. “We’re now mining that resource and taking it forward for patients,“ he says. “And we’re sharing our data with the scientific community because it could help advance the entire field.”
 VIDEO
VIDEO
Beyond the parts list
The team worked with 398 patient-derived cancer cell lines representing a variety of devastating malignancies. The lines had all been genetically sequenced and characterized by Novartis and the Broad Institute of MIT and Harvard as part of a joint research program called the Cancer Cell Line Encyclopedia. Sequencing had revealed gene mutations in the cancer cells, and a molecular “parts list” of their inner machinery.
“But it didn’t tell us much about which genes are functionally significant,” explains Rob McDonald, a senior researcher in oncology at NIBR and a co-corresponding author of the Project DRIVE paper. “We wanted to identify vulnerabilities – the genes and proteins that cancer cells care most about.”
To find them, the team delivered gene-silencing strands of RNA into the cancer cell genomes with a viral vector. This method, called RNA interference (RNAi), is designed to be gene-specific. If a cell dies or stops growing after a targeted gene is turned off with RNAi, researchers flag it as crucial to the cell’s survival.
In all, nearly 8,000 genes – or about a third of the entire human genome – were screened during the project. Both normal and mutated genes were selected for screening, with a focus on genes representing potential drug targets.
Meanwhile, the research proceeded on a vast scale: The global team used 20 different RNAi reagents per gene across all the cell lines that were tested. RNAi molecules sometimes hit random targets in the cell and produce confounding results. But by adding more reagents, the team increased confidence in its ability to identify the effects of silencing individual genes accurately. Moreover, this robust approach helped guarantee that Project DRIVE would generate reproducible findings. Ultimately, the researchers cultured trillions of human cancer cells, using more than the surface area of a professional basketball court to complete the screen.
By then combining Project DRIVE’s functional output with molecular information about each cell line, researchers mapped out the cancer cells’ genetic dependencies. McDonald co-led a team of scientists and bioinformaticians who were tasked with analyzing and making sense of the data. “Our goal was to find out why lung cancer, for instance, cares about a particular gene,” he says. “Maybe the gene is highly expressed in lung cancer, or maybe it’s mutated, or perhaps there’s a totally novel relationship between that gene and the disease that we’re not aware of.”
The team looked for molecular features that might predict sensitivity to gene silencing effects. These features include mutations, genomic amplifications and deletions, chromosomal translocations, and changes in gene expression. If knocking down a particular gene is harmful only to a specific subset of the screened cell lines, “then we might ask, ‘What do each of those cell lines have in common?’” McDonald explains. “If they share a feature that the other cells don’t, then you can form a hypothesis – specifically that the feature predicts sensitivity to gene silencing that might have therapeutic implications.”
Making sense of gene “hairballs”
Genes and the proteins they encode operate in dense complexes (often referred to as “hairballs”) that scientists must probe. “When we zoom into these hairballs, networks of individual signaling pathways start to emerge,” McDonald says. “It’s possible to see how different genes and proteins relate to each other in functional hubs.” These hubs might include targets with similar functions, or targets that operate in the same molecular complexes or pathways.
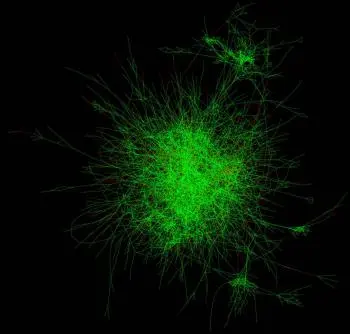
But cancer is a complex disease that must be studied from many different angles. So the team is sharing its findings and making this data freely available to outside researchers. Using a publicly available web tool, researchers can evaluate pathways and targets of interest to them across all 398 screened cell lines. Or they can access the raw data and develop new algorithms to study targets in other ways.
“This project has informed our thinking about the targets we want to go after to cripple cancer cells,” says Jeff Engelman, Global Head of Oncology at NIBR. “The findings are extremely robust. I don’t know of any other biotech or pharmaceutical companies that have undertaken such a comprehensive effort. The significance of this research is not just for the medicines we make here at Novartis, but for the advances that this will enable throughout the global biomedical research community.”
Main image: metastatic melanoma cells by Julio C. Valencia/NCI Center for Cancer Research
Novartis shares data from Project DRIVE experiment to advance #cancer research.