The liver disease called NASH (non-alcoholic steatohepatitis) is stealthy. Patients don’t have symptoms until the latest stages of the disease, when it almost immediately becomes life threatening. It sneaks by doctors, too, since the disease can only be confirmed with a liver biopsy, an invasive procedure reserved for the seriously ill.
NASH is also hard to treat. It’s a bit like four diseases in one, typically starting with the buildup of fat in the liver, called steatosis, but also involving inflammation, liver cell death and scarring, called fibrosis.
Because the disease is so complex, Novartis researchers are seeking to develop experimental combination therapies for NASH straight out of the gate, before there is even a single drug approved for the disease. The first Novartis combination therapy trial for NASH is expected to begin this year, combining an in-house agent called tropifexor (also known as LJN452) with a compound from Allergan called cenicriviroc that is in Phase 3 studies. Other combination trials are planned to follow. This collaborative effort is part of a larger strategy looking to bring potential therapies to patients with a range of complex liver diseases.
“It seems unlikely that we’ll find one drug that treats all of the components of NASH effectively,” says Bryan Laffitte, the brains behind tropifexor and a director at the Genomics Institute of the Novartis Research Foundation. “To get the best results and resolve the disease – which is what we want – we need to address it using combinations of drugs.”
Since no medical interventions for NASH are available, doctors recommend weight loss. The few compounds that have so far been tested on their own in clinical trials for NASH have improved the livers of fewer than half of patients. “A similar story unfolded for hepatitis C virus,” says Eric Hughes, Head of the Immunology and Dermatology unit at Novartis. “The first drugs helped a small percentage of people, but in the end it was a combination that slayed the dragon.”
NASH begins as non-alcoholic fatty liver disease (NAFLD), and is typically related to other conditions, including obesity and diabetes. Estimates suggest that NAFLD affects one-quarter of the world’s population, and about 25% of those patients have NASH. NAFLD also affects younger people, including children. When NAFLD progresses to late-stage NASH, the only life-saving option is a liver transplant. In the United States, NASH has become the leading cause of liver transplants in people under 50.
 VIDEO
VIDEO
A start to stopping NASH
NASH was a dim blip on the medical radar when Laffitte came to Novartis in 2005. So much so that he didn’t have the disease in mind when he started investigating the farnesoid X receptor (FXR), a protein in a class of receptors that control the execution of entire genetic programs of cellular behavior and also make excellent drug targets.
He and his team soon recognized its role in regulating the flow of bile acid in the liver, and also its potential to treat NASH. In NAFLD and NASH, fat builds up in the liver and leads to inflammation and scarring that blocks the flow of bile acid. In turn, excess bile acid causes more inflammation, scarring, and cell death. FXR naturally detects bile acid and launches protective measures, though it can’t keep up as the disease progresses.
The researchers set out to harness and amplify FXR’s built-in ability to handle excess bile acid in the liver. This effort led to the discovery and development of tropifexor. So far, research results suggest that tropifexor could slow the buildup of fat in the liver and potentially reverse liver scarring. Trials are in progress in humans with NASH.
Because tropifexor does not address all aspects of NASH, the researchers started to wonder if they might see even better results with a cocktail. “It just makes sense to combine things that have different mechanisms of action to get at the disease from multiple angles,” says Sujata Vaidyanathan, who is responsible for clinical trials in NASH patients as Senior Global Program Head in Global Drug Development at Novartis.
Finding the right partner
The team started looking for a potential partner compound that might reduce inflammation in the liver, something tropifexor affects the least. Publications about C-C chemokine receptors (CCR2/5) grabbed their attention. Inhibiting CCR2/5 on certain cells in the liver could reduce inflammation. “This seemed like a really great option to combine with our FXR agonist,” says Laffitte. “But that was hypothetical. We needed to put the two together and see the outcome.”
The team launched a study in mice that tested tropifexor alongside cenicriviroc, an experimental drug that inhibits CCR2/5. The mice in the study modeled human symptoms of NASH and were fed a high-fat diet. They were given a placebo, one of the two compounds alone, or both compounds together.
The researchers used the NAFLD activity score (NAS) to measure the effectiveness of the interventions. The NAS includes measures of steatosis, inflammation, and precursors of cell death. “The nice thing about that score is that it captures all of the components of the disease except fibrosis,” says Laffitte.
Healthy mice had average NAS scores of 0, while untreated NASH mice had average scores of 4.8. Mice taking either agent alone had scores of about 3. Those taking the drugs in combination had scores of 1. Fibrosis, which was measured separately, was reduced by about the same amount with both agents alone and in combination.
The results suggest that this combination might have a bigger impact on NASH than either compound alone. Based on this evidence, Vaidyanathan’s team coordinated with Allergan to launch a clinical trial of the combination in NASH patients.
While this is the first combination therapy Novartis is testing for NASH, it likely won’t be the last. Another trial is planned to test a combination of tropifexor with a drug developed by Novartis and Conatus Pharmaceuticals called emricasan (also known as VAY785) which addresses inflammation and liver cell death. Combinations of internal experimental therapies are also in the works. “In the long term we’d like to have a range of compounds we can combine,” says Vaidyanathan.
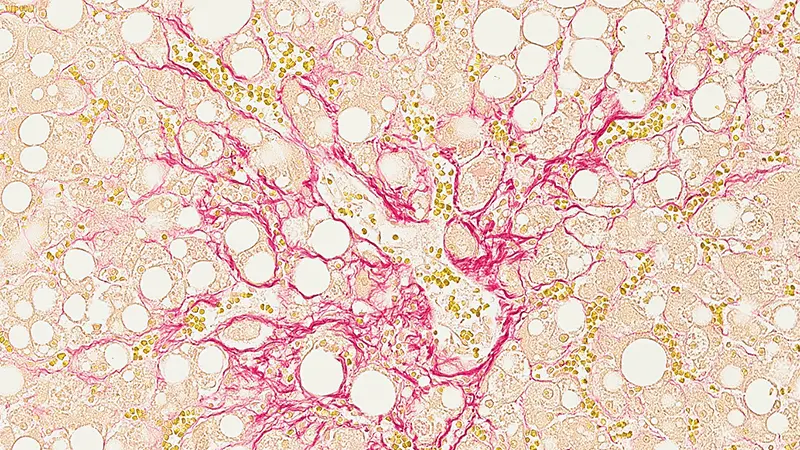
Main image: An estimated one in four people worldwide has fatty liver disease, a silent disease that can progress to nonalcoholic steatohepatitis (NASH), which is serious and can lead to liver failure. Image by Novartis.



