When tackling a disease, patients are usually offered treatments that prolong or improve life. Sadly, for some patients, there comes a point where all approved therapy options are exhausted.
One solution may be enrolment in a clinical trial, allowing patients to receive promising medicines as they are being tested. When this is not available or the patient is not suited, another option is access via ‘compassionate use’ (CU) programs, at Novartis referred to as ‘Managed Access Programs’ (MAPs), which enable access to locally unlicensed medication (generally free of charge) for patients with serious or life-threatening medical conditions.
An established approach
Programs of this kind are not new, but the recent COVID-19 pandemic has placed a spotlight on the value of CU as healthcare systems sought ways to better prevent, treat and manage a previously unseen infection, by gaining rapid access to promising investigational therapies based on emerging clinical trial evidence.
As well as benefiting patients and healthcare professionals, CU provides an opportunity to collect real-world data, which can ultimately help speed medicines approval. This is especially important in rare diseases where there are often no or very few treatment options.
“The right thing to do”
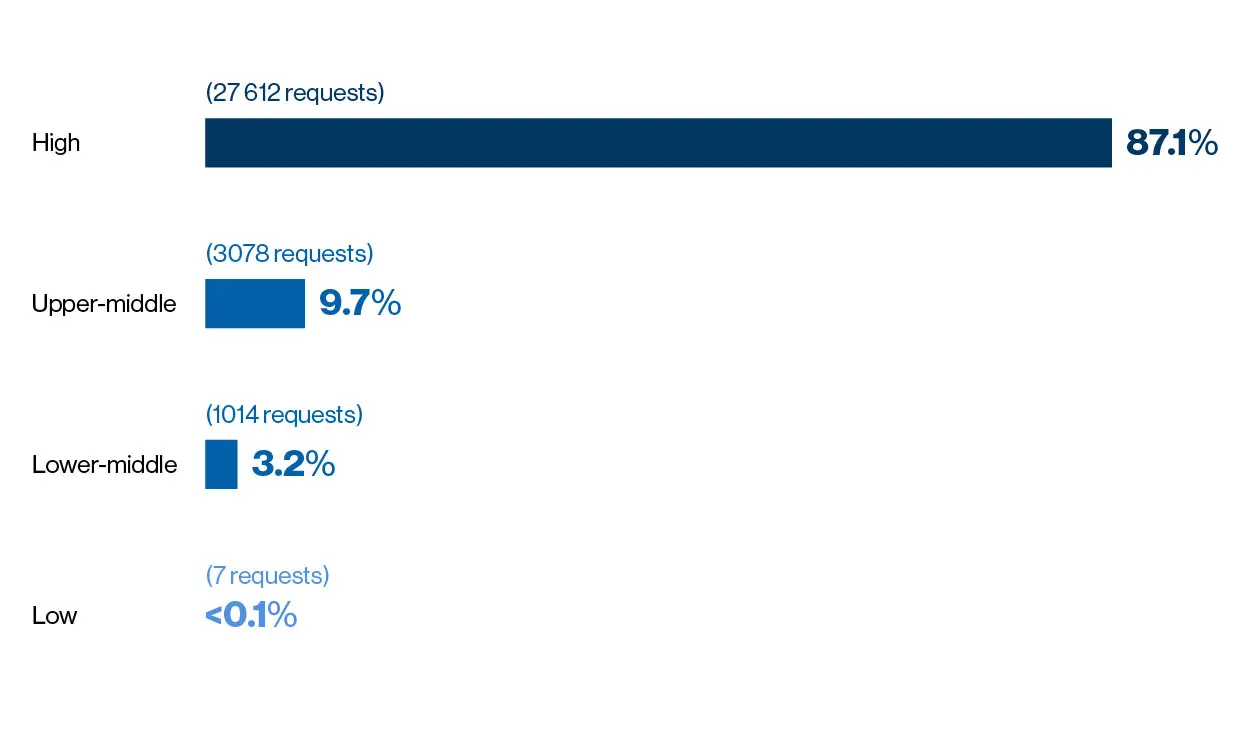
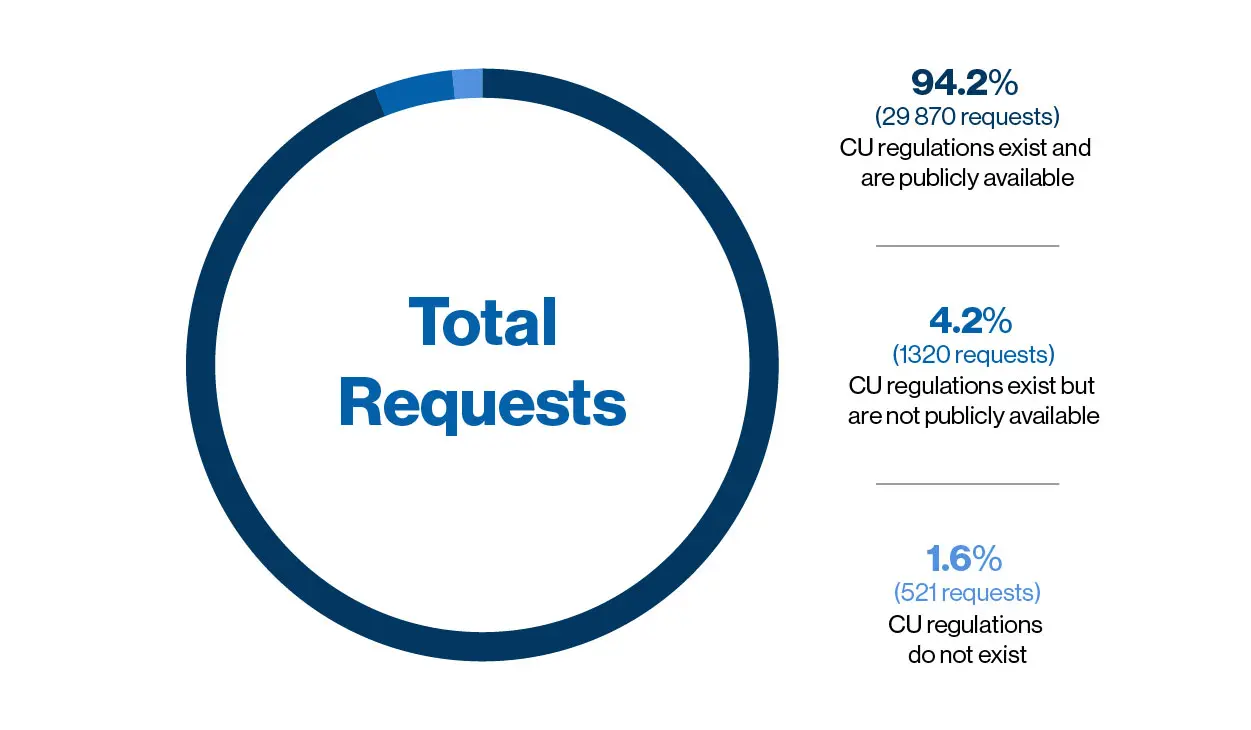
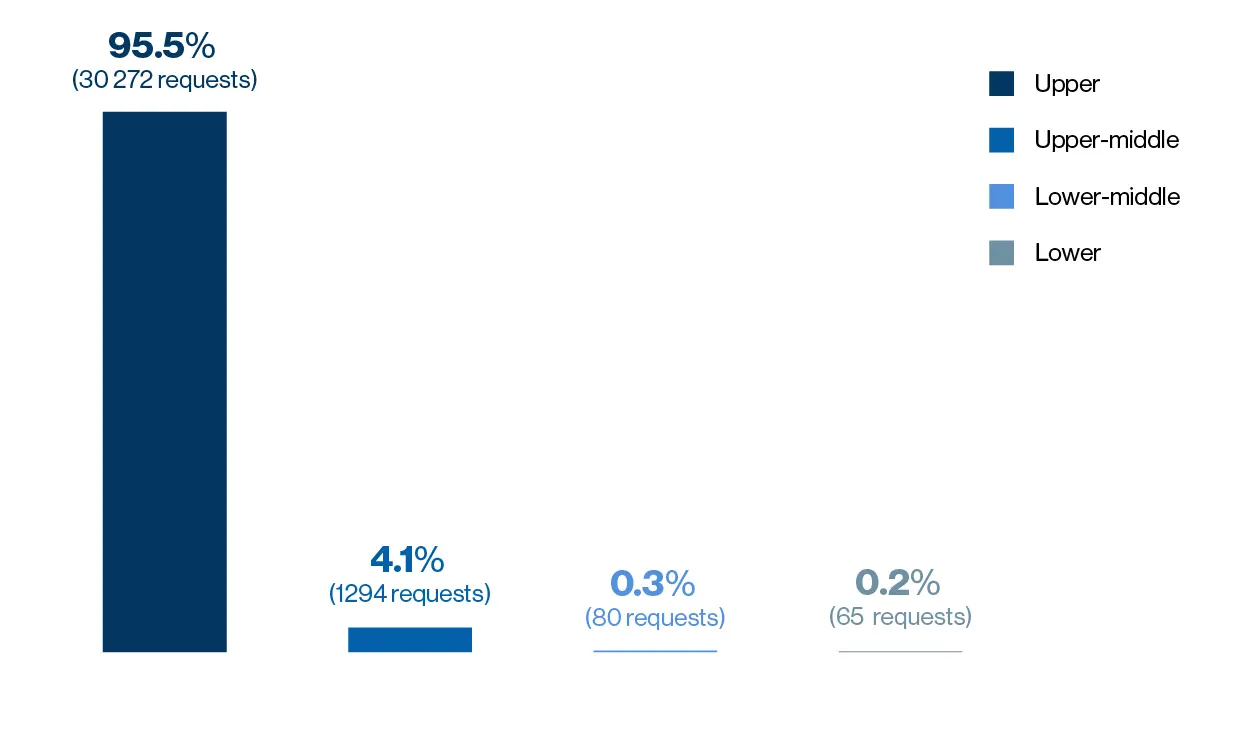
Novartis receives an average of 10,000 CU requests a year, with an approval rate of around 95 percent for the reviewed requests. Paul Aliu, who heads the Global Governance Office in the cross-divisional Chief Medical Office at Novartis, puts this high approval rate down to a genuine desire to ‘do the right thing’.