When a fire alarm sounds, first responders race to the scene. Firetrucks follow, hoses at the ready. Water doesn’t flow, however, until the first responders locate the fire.
A similar process takes place in the body in response to an injury. The immune system detects a problem and sends out its first responders, a flow of inflammatory signals, to secure the area before letting in the “firefighters.”
In cancer, however, the tumor confuses the first responders by keeping the alarm bells ringing. The body responds with more and more inflammatory signals. Meanwhile, the fire fighters of the immune system, T-cells, sit idle, waiting for permission to take action.
“It’s basically a miscommunication,” says Pushpa Jayaraman, a scientist in the Exploratory Immuno-oncology group at the Novartis Institutes for BioMedical Research (NIBR).
Novartis researchers have found a potential way to clear up this miscommunication. By blocking a molecule called interleukin-1 beta (IL-1 beta), they aim to lift the blockade created by the confused first responders and enable the firefighters of the immune system to begin attacking the tumor. The research is part of a larger effort to identify new ways to block chronic inflammation – and potentially unlock a powerful new set of tools to help treat a range of diseases, from Alzheimer’s disease to autoimmune disorders to cancer.
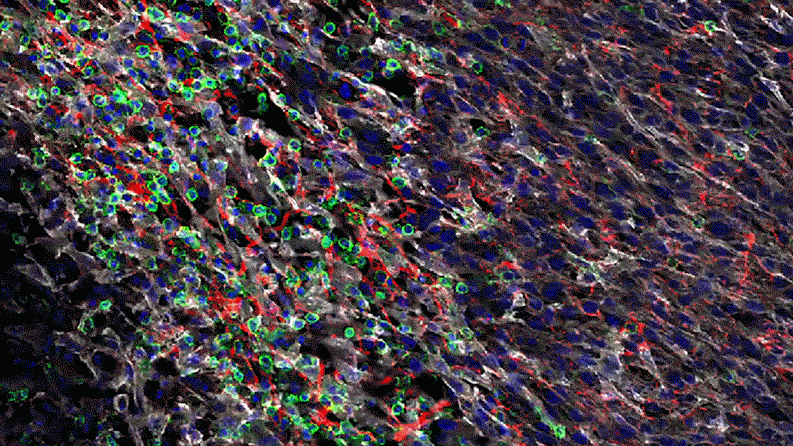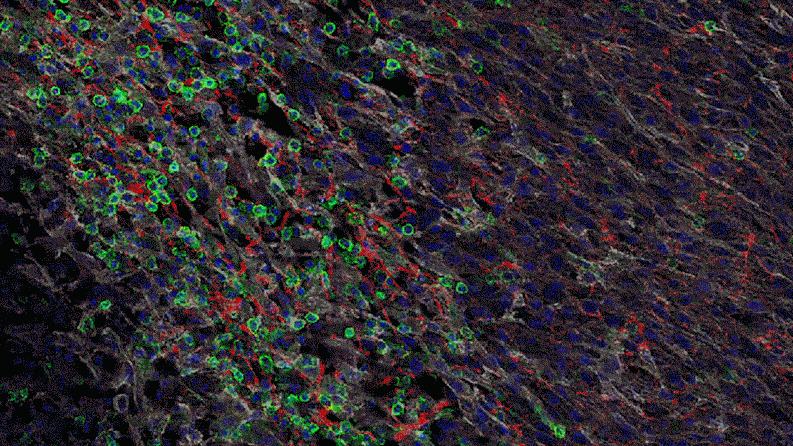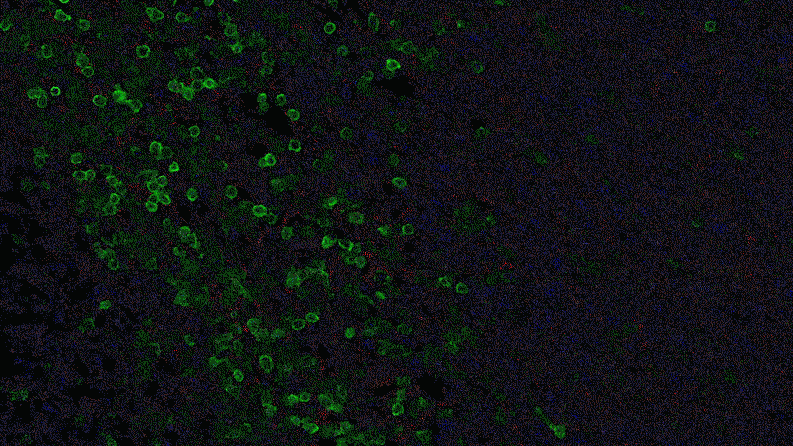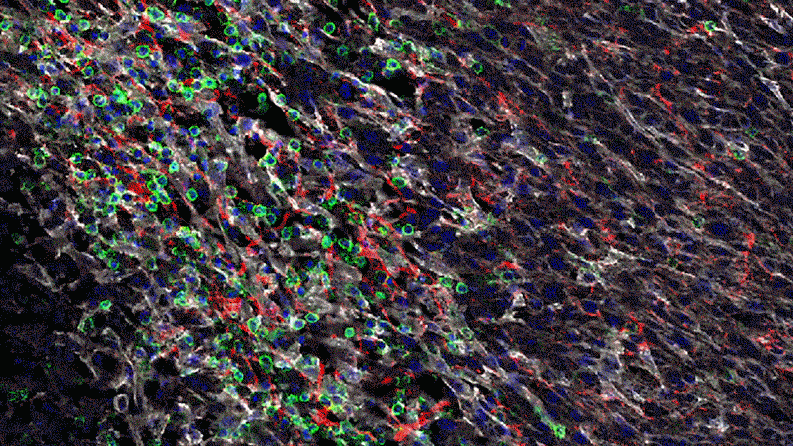
Inflammation that lingers has long been associated with chronic diseases, including cancer. Elevated levels of IL-1 beta are linked to the growth and spread of tumors, so quashing IL-1 beta could potentially help fight cancer. “This could lead the next wave of immuno-oncology,” says Lauren Abrey, Global Franchise Head, Solid Tumors and Rare Diseases, at Novartis.
Novartis has launched several clinical studies of canakinumab, a monoclonal antibody that blocks IL-1 beta. The studies are testing the experimental agent alone and in combination with other medicines in patients who have different stages of non-small cell lung cancer, the most common form of lung cancer. Testing in human clinical trials is required to establish the safety and efficacy of this approach.
Despite advances in medicine, patients with non-small cell lung cancer have limited treatment options and survival times, especially if they are diagnosed after the cancer has spread.
 VIDEO
VIDEO
Clinical evidence in lung cancer
The idea that inflammation could promote cancer is more than a century old. It wasn’t until 2017, however, that the first evidence in humans showed that blocking inflammatory signals could change the course of cancer and potentially slow its growth. This evidence came from an unexpected place: a Novartis clinical trial focused on cardiovascular disease.
The trial had tested canakinumab in over 10 000 people with heart disease. It turns out that this patient group also had a high risk of lung cancer. To participate, patients had to be free of a cancer diagnosis, but they also had to have high levels of a marker of inflammation called C-reactive protein. This marker is associated with increased lung cancer risk.
The immune system thinks it needs to tend to a wound. But instead of wound healing, it ends up protecting the tumor.
Pushpa Jayaraman, NIBR immuno-oncologist

After the trial ended, investigators performed a hypothesis-generating exploratory analysis. They found that patients in the study taking canakinumab were 77% less likely to die from lung cancer and 67% less likely to have lung cancer compared to those not taking the drug, suggesting the experimental drug could potentially play a role in lung cancer treatment. The findings were published in a medical journal called The Lancet.
These findings piqued the curiosity of NIBR researchers. “We wanted to learn more,” says Catherine Sabatos-Peyton, a director of the NIBR Immuno-oncology group.
Remodeling lung tumors with anti-inflammatory tools
To start, a team of Novartis investigators did a deep dive into the cardiovascular trial data by looking at blood samples taken from patients during the course of the study to make sure there wasn’t something else going on to alter the course of cancer.
Their analyses confirmed that changes directly associated with the IL-1 beta pathway were the main difference between those who developed or died from lung cancer and those who did not. Other inflammatory markers had not changed in relation to lung cancer risk.
Now the team wanted to know what was happening inside the tumor to change the course of cancer so dramatically.
In a series of studies in mice, Jayaraman found that blocking IL-1 beta changes the cellular makeup of the tumor. In the presence of inflammatory signaling driven by IL-1 beta, the tumor is awash with cells with the power to suppress the body’s natural defenses. That is, they could prevent the firefighters from dousing the fire. For instance, specialized immune cells infiltrate the tumor and dampen the activity of T-cells, which normally would attack the tumor.
“The immune system thinks it needs to tend to a wound,” says Jayaraman. “But instead of wound healing, it ends up protecting the tumor. It gives the tumor a safe space to grow. Blocking IL-1 beta could break that cycle.”
Jayaraman observed that when IL-1beta is inhibited, the number of those suppressor cells drops. At the same time, more immune cells equipped to attack the cancer, such as T-cells, invade the tumor. “By inhibiting IL-1 beta, we’re potentially stripping away a protective shield,” says Jayaraman, who presented data from these studies at the World Preclinical Congress in July 2019.
Novartis has launched a series of clinical trials testing the effects of canakinumab on non-small cell lung cancer. The largest trial, CANOPY-A, is expected to recruit 1 500 patients who have had surgery to remove their lung cancer, and will test the effects of canakinumab alone versus placebo. The other trials will test canakinumab in combination with immuno-oncology agents designed to lift the brakes on anti-tumor immune activity, and/or chemotherapy agents. Trials are exploring the use of anti-IL-1 beta therapy before surgery to remove the cancer, after surgery, and in cases where the cancer has spread.
Because chronic inflammation is associated with many diseases, the Novartis team is investigating a range of approaches to modulating inflammation. “This could be the beginning of a new approach to treating a wide range of diseases, though there’s much to learn,” says Sabatos-Peyton.
Main image: Photo credit, Fidelis Onwubueke.
Video by Dwayne Quimby.
This story was updated in December 2019 with additional video content.
