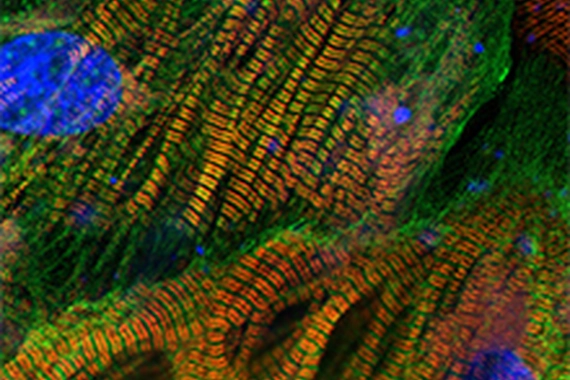
Cardiovascular and metabolic diseases
Our Cardiovascular & Metabolic Diseases team works to better understand the root causes of cardiovascular and metabolic diseases, and discover and develop therapies against relevant targets.
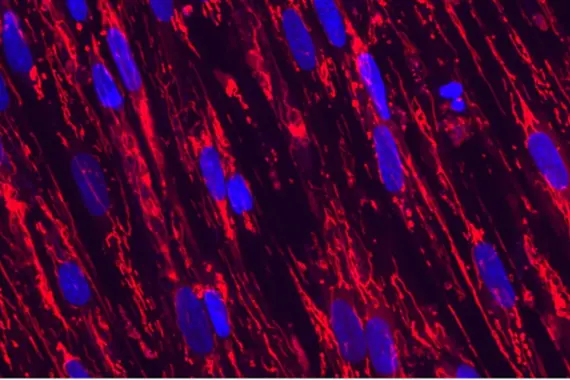
Diseases of aging and regenerative medicine (DARe)
The DARe group is focused on elucidating and targeting the molecular mechanisms underlying diseases of aging and developing potential regenerative pharmacological interventions that restore tissue integrity and function.

Exploratory disease research (DAx)
DAx catalyzes and drives transformative therapies for emerging, high-potential indications. Current research focuses on liver and kidney diseases, and fibrosis.
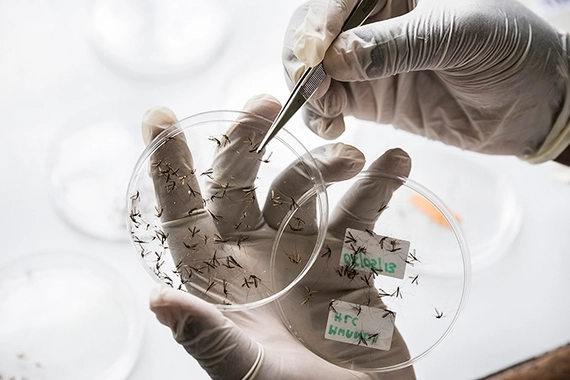
Global health
The Global Health Disease Area (DA) is discovering novel medicines to help control and, ideally, eliminate diseases impacting some of the world’s most vulnerable and underserved populations.
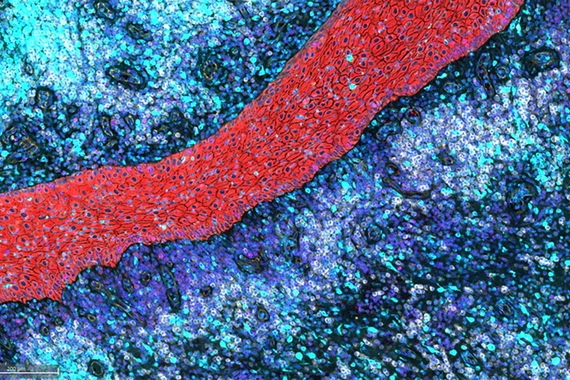
Immunology
The Immunology group is developing transformative therapies, including small molecules and biotherapeutics, that target the root cause of disease by modulating the immune system to restore health.
Neuroscience
Our neuroscience research team works to discover transformative therapies that address the underlying cause of neurodegenerative, neurodevelopmental and neuropsychiatric diseases to dramatically improve the lives of patients.
Oncology
Our Oncology team is developing a robust portfolio of treatments that destroy tumors selectively and strip away their defenses.