At Novartis we hold ourselves to high ethical standards. Not only our Code of Ethics outlines the values and behaviors for our associates but also our Third Party Code (PDF 0.4 MB) defines the standards we require our external partners to adhere to.
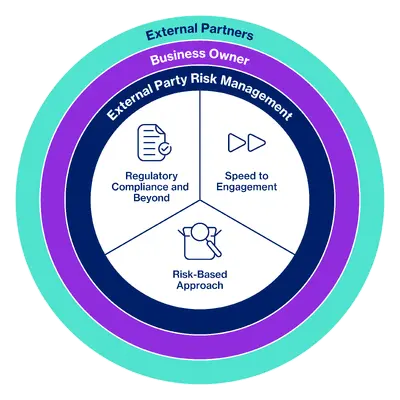
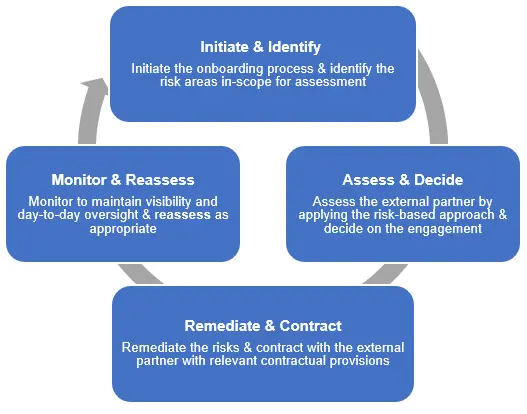
Novartis promotes the societal and environmental values of the United Nations Global Compact and United Nations Guiding Principles on Business and Human Rights to its external partners and uses its influence where possible to encourage their adoption. To achieve this, Novartis has developed an External Partner Risk Management (EPRM) framework that supports the assessment and management of risks related to the engagement of external partners. By understanding the exposure and managing it effectively, we protect our patients, the company, and shareholders against adverse impacts such as regulatory, financial, or reputational damage.
EPRM is an integral part of the Ethics, Risk and Compliance (ERC) function at Novartis.