On a spring day in New York City in 1891, a young doctor named William Coley loaded a syringe with virulent, potentially lethal bacteria. He then thrust it into an inoperable tumor lodged in a patient’s neck. Coley repeated this procedure over several months, until one day, a high fever gripped the patient. Soon, the tumor began to shrink. In a few weeks, it disappeared. There was no sign of the tumor for the better part of a decade.

More than a century later, Coley is recognized as the first to systematically test the idea of treating cancer by harnessing a patient’s own immune system, an approach known as immuno-oncology, or cancer immunotherapy. This concept is among the most promising in medicine, with the potential to give long-lasting control over once-intractable tumors.
One of the same challenges that vexed Coley, however, continues to loom large: Only some cancers, typically ones that form solid tumors, respond to current immunotherapies. For patients with other cancers, such as blood cancers, immunotherapy options are extremely limited.
This is a puzzle that must be solved piece by painstaking piece. But with every new insight, researchers around the world, including at Novartis, take a step closer to unlocking the potential of immunotherapies for more patients and for more cancers.
Our best defense against cancer
Coley tested variations of his bacterial approach over several decades. Despite his best efforts, he found it impossible to predict who would respond or to explain why. His methods fell into disrepute, until the world entered the modern era of biomedicine.
“Today, we understand that our immune systems are our best defense against cancer. All patients who develop cancer, at some level, have a failure of the immune system,” says hematologist-oncologist David Steensma, who leads early-stage hematology research at Novartis. “Cancer cells trick the immune system into thinking that they belong there, that they are healthy cells and don’t need to be eliminated.”
Fundamentally, the immune system is responsible for recognizing what belongs in the body and eliminating what does not. To do so, immune cells have to decide whether to activate against a potential threat or to stand down.
To create immunotherapies that can work for more patients, we need to better understand the interactions between cancer and different components of the immune system
Renaud Capdeville, global program head for hematology at Novartis
In the early 1990s, researchers identified a critical protein involved in this decision, which acts as a molecular brake on the immune system. Without it, immune cells erroneously attack healthy tissues.
Immunologist James Allison, who would later share in the 2018 Nobel Prize, asked a transformative question: Could this discovery be useful against cancer? His efforts, as well as those of fellow Nobel laureate Tasuku Honjo and many scientists around the world, revolutionized oncology.
 VIDEO
VIDEO
A revolution in oncology
They showed that cancers could indeed co-opt proteins that can turn off the immune response, called checkpoints, to trick immune cells into standing down. More importantly, blocking these proteins with medicines – thus releasing the brakes – allows immune cells to attack tumors, often with remarkable efficacy.
Based on these findings, new immunotherapies emerged that now serve as foundational tools in the fight against cancer. Coley’s vision was beginning to come to fruition.
Current immunotherapies, however, only work in a subset of cancers and a subset of patients. For scientists at Novartis and across the globe, one of the most critical needs in medicine today is to expand the number of people who can benefit from immunotherapies.
One of the key insights in this endeavor is that the immune system has many molecular checks and balances that determine when it ramps up or slows down – any of which can be exploited by cancer cells to evade the immune system.
Uncovering these myriad tricks is a priority for researchers because once they are exposed, counterstrategies can be created.
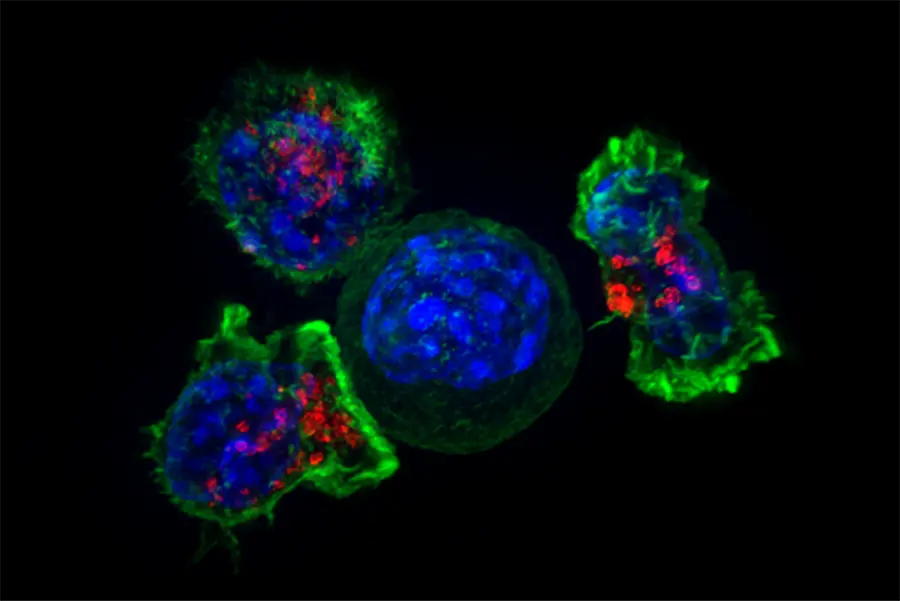
Activating the immune system
In some blood cancers, for example, researchers found that the immune system appears to be lulled into an abnormal slumber. Intriguingly, certain immune cells, called myeloid cells, express high amounts of a checkpoint-like protein that has been linked to dysfunctional immune activity in these diseases – a clue to the trick being employed.
Just as releasing molecular brakes can activate immune cells in other cancers, Novartis scientists are asking whether these checkpoint-like proteins play a special role in these diseases, and whether blocking them can help reawaken the immune system.
Similar efforts are ongoing to probe the behavior of other checkpoints and proteins that cancers use to evade the immune system.
Because many cancers use certain tricks but not others, this work may enable scientists to activate the immune system in new ways, particularly in diseases with currently limited immunotherapy options, such as blood cancers.
“To create immunotherapies that can work for more patients, we need to better understand the interactions between cancer and different components of the immune system,” says Renaud Capdeville, who is global program head for hematology at Novartis. “These insights are critical if we are to successfully solve this puzzle.”
At Novartis and elsewhere, scientists are also exploring new ways to combine immunotherapies with other cancer treatments, as well as many other strategies to unleash the immune system. With each new discovery, they unlock potential new approaches that may help more patients.
More than a century ago, most considered the idea of immunotherapy eccentric, at best. But today, we’re getting closer and closer to finally solving the puzzle that Coley first began working on in 1891.