Today, the power of data analytics is informing strategies and business practices across industries. Football clubs are chasing players with specific skills to strengthen a side’s qualities, and Formula 1 teams are analyzing every turn of the wheel to squeeze out milliseconds to win races. The promise of big data and advanced analytics, which has been fueled by a quantum leap in computing over the past 10 years, is changing industries and giving rise to new ones. Self-driving cars, Google Assistant and even Amazon online retail would not be possible without powerful computing techniques such as machine learning and cognitive computing.
These new developments have prompted many experts to believe that the world is on the brink of a fourth industrial revolution, which is set to impact every fiber of society. Industry 4.0, as it is often referred to, is fueled by hopes – as well as fears – that collecting and cleverly analyzing big data sets will lead to new and unexpected knowledge that until recently was difficult or even impossible to generate.
A quantum leap in thinking
Novartis has joined this race. “We are experiencing a data science revolution that we need to harness,” says Dr. Luca Finelli, who is leading the Predictive Analytics & Design group within Global Drug Development. His team has developed a new platform called Nerve Live, which uses the latest advances in computing technology and is designed to help Novartis leverage its huge data pool. “In reality, we are a data company,” Dr. Finelli explains. “We are used to generating and working with huge amounts of data, analyzing it, and using this knowledge to research and develop new therapies. If we are able to bring our data into one place and tap into the latest computing technologies, we can generate new insights that in the past were difficult to obtain because our data was locked in silos.”
The idea of digital technologies becoming ubiquitous with broader leadership in healthcare has become ingrained into Novartis GDD
Efforts to build an advanced analytics platform started in 2015, and following the creation of the Global Drug Development, or GDD, organization in 2016 brought data related to the past and present global clinical trial programs of Novartis, numbering close to 500, under one roof for the first time.
Over the past few years, digital healthcare has grown exponentially. The amount of data collected by the industry has increased by almost 50% every year. It is set to continue its substantial rise as computing services become more powerful and cost-effective. Just take genome sequencing: While it took about 10 months and cost about USD 10 million to sequence a human genome in 2007, the same process today takes about 24 hours and costs in the range of USD 1 000 or less. Thus, the idea of digital technologies becoming ubiquitous with broader leadership in healthcare has become ingrained into Novartis GDD. “It was not an easy journey,” recalls Dr. Finelli. “Our team had to rethink how we integrate, analyze and use our data.”
Building a big data platform
First, the team had to build a so-called data lake. As part of this effort, the team had to replicate the operational data of GDD into a single system, which today sits on a Novartis-owned cloud. Even this first step was far from easy because the proposed platform architecture was not a Novartis standard. The data also had to be integrated because it was locked in domain-specific silos owned by different teams, displayed inconsistencies and was difficult to access.
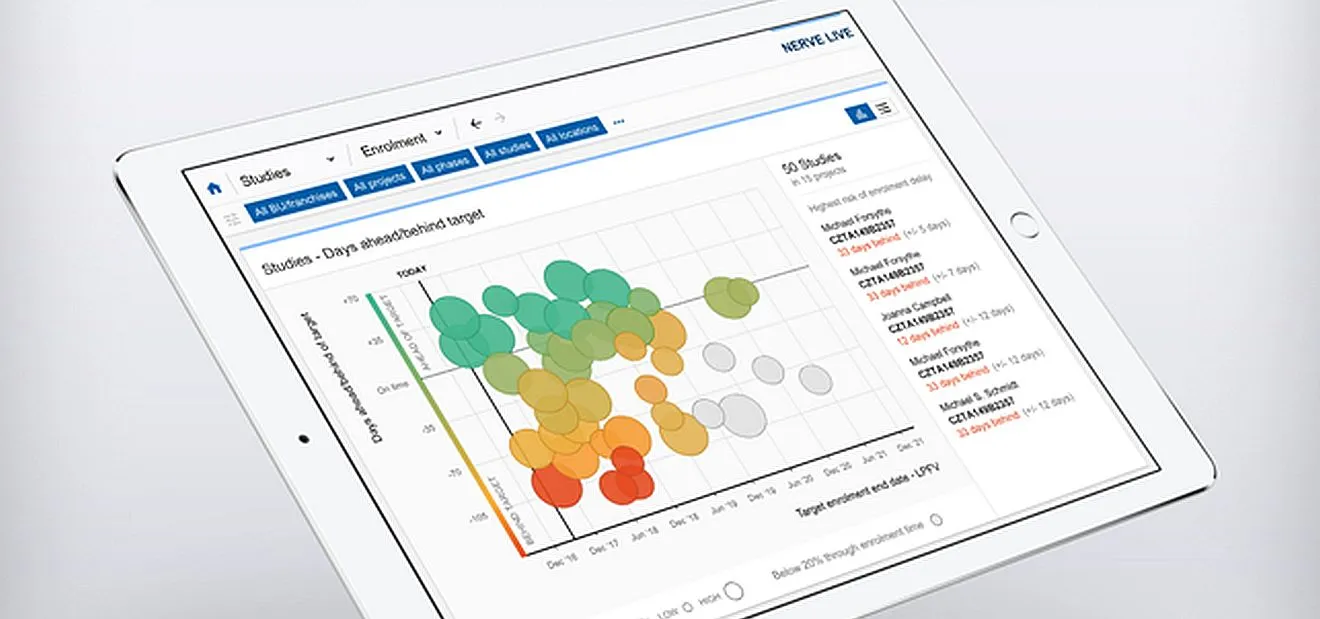
Next, the team worked on generating an advanced analytics engine to process the data and distill new, actionable insights from it. “This is the brain of Nerve Live,” Dr. Finelli says. “With this system we can now apply the latest machine learning or cognitive computing algorithms to answer questions that have business value and bring our data to life.” In a nutshell, these new algorithms can use past and current data to make performance predictions that could enable users to take preventive corrective action and make better informed and faster decisions.
Step 3 was to start designing user modules that would make the predictive analytic insights intuitive and bring them to users to add value in business decisions. This step is open-ended. So far, five modules have been created and the team is already working on the next wave. The Trial Footprint Optimizer helps GDD teams plan and simulate country allocation scenarios for clinical studies, such as choosing the best sites, tracking enrollment, and predicting patient enrollment curves. Another module serves as a “control tower” for clinical studies, called Sense. Sense is designed to be operated from a central room equipped with large wall screens and dedicated workstations. This platform will support the evolving portfolio of clinical studies in real time by predicting study risks and their drivers to enable preventive maintenance and remediation. Sense further provides a setting for actionable collaboration, using data to enable transparent conversations between teams around the world running Novartis clinical trials.
Future developments of Nerve Live could include helping to better manage resources and using predictions to plan the supply of Novartis medicines to medical centers. With this growing portfolio of integrated modules, the team is building a cohesive ecosystem that could enable the organization to efficiently generate benefits along the entire value chain.
Let the data speak
Besides the ease of use and expected productivity gains, Dr. Finelli believes the transparency that comes with big data analytics and especially Nerve Live will be one of the biggest change drivers in the years to come. “In the future, we will need to understand the story that our data is telling us, and not the other way around. We will need to shift from a knowledge culture to a learning culture.”
While the data will be transparent for everyone to see, powerful technologies such as machine learning and cognitive computing, which – for example – enable computers to learn a task without being explicitly programmed, can extract information that previously remained hidden. In many ways, the cultural change could be similar to the one seen in baseball, where scouts in the past followed their intuition to pick players instead of looking at a player’s data in more detail, as is the case today.
In the realm of clinical trials, teams would not choose hospitals based on gut feeling or proximity. Rather, they would consult the Trial Footprint Optimizer and check whether a hospital has the capability to run a trial according to the study plan requirements. “This will take some time before it is fully grasped,” Dr. Finelli says. “But I believe that, in view of the substantial productivity gains we can achieve with this data-driven transformation, our teams will adapt quickly.”



