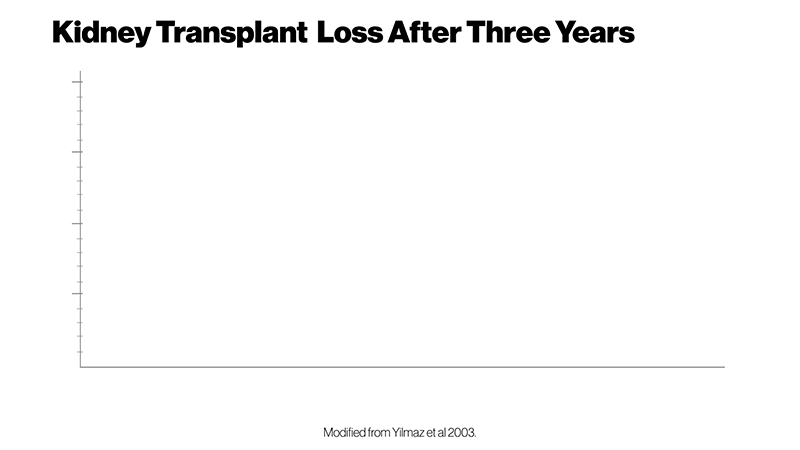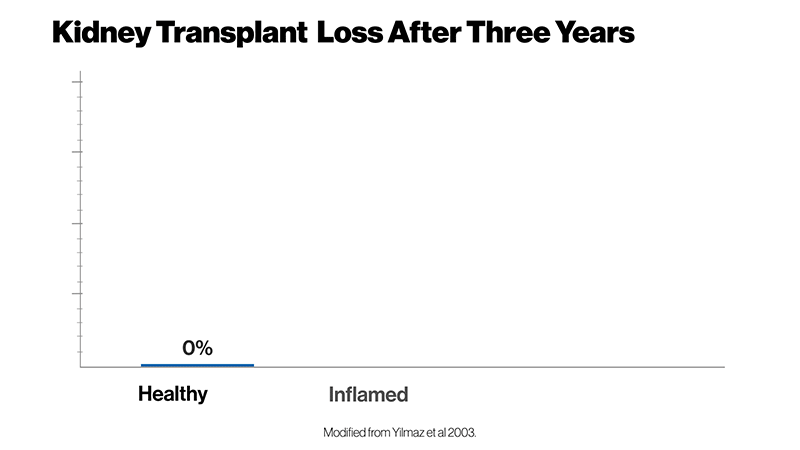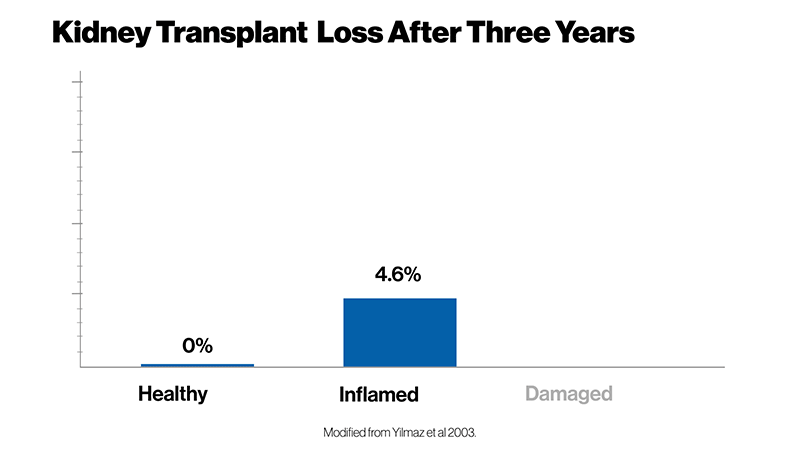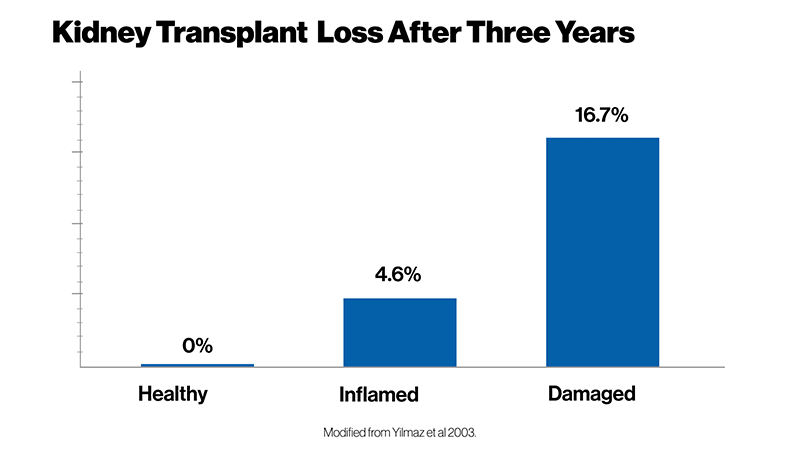The ability to transplant an organ from one person to another is an incredible feat of medicine. It turns out, however, that transplanted organs aren’t as durable as one might think.
The very medicines that keep the body from rejecting the organ also slowly destroy it. A donated kidney, for example, may last five years. Most don’t last 10. Some patients end up back on dialysis, visiting a clinic multiple times a week to have waste filtered from blood mechanically while waiting for a second donated kidney – in a queue that lasts between three and five years in the US.
Novartis researchers think they have an approach to transplant support that, if successfully developed, could make the promise of durable transplants a reality for many patients.