
CAR-T cell therapy: The living drug1
CAR-T cell therapy is a targeted, personalised therapy that contains patients’ autologous T cells reengineered to fight cancer. This living drug continues to exist in the body and combat cancer long after its infusion.
| Personalised: Establishes individual treatments for individual patients1 | |
| Reengineered: Harnesses the power of our existing defense mechanisms to fight cancer1 | |
| Persistent: Provides hope for long-lasting efficacy to relapsed or refractory patients who need it most1 |
The structure of CAR-T cells1
CAR-T cell therapy contains patients’ autologous T cells that are modified to express the CD19 chimeric antigen receptor (CAR), which helps eliminate B cells, including those that are malignant. Current CAR-T cell therapies contain CARs composed of five parts. Please see the diagram and accompanying text below to learn more about the function of each component.
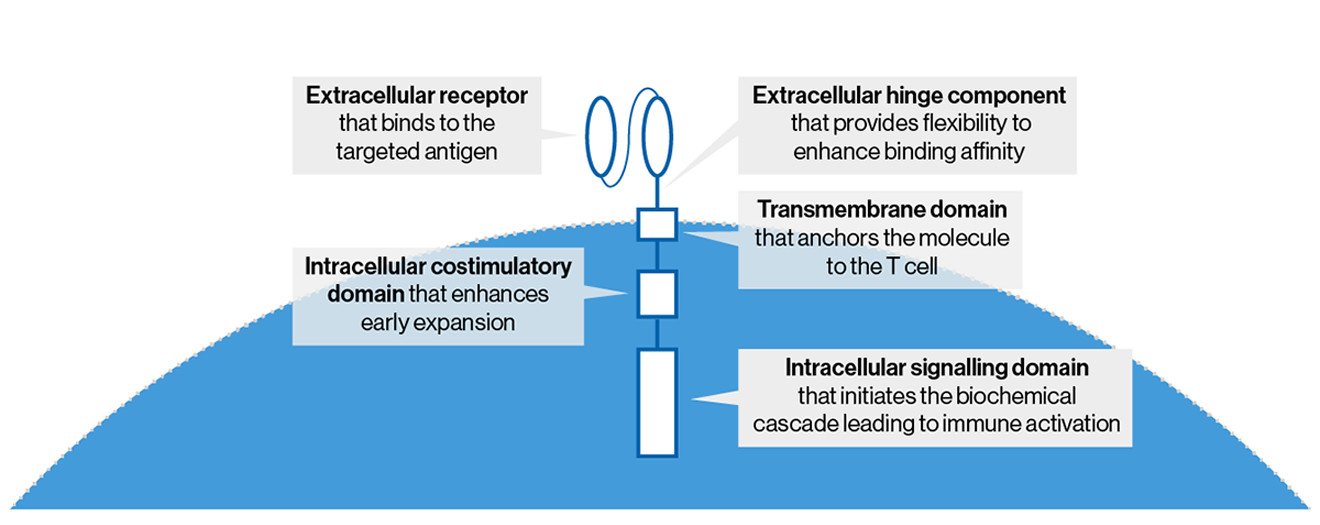
- Extracellular receptor that binds to the targeted antigen
- Extracellular hinge component that provides flexibility to enhance binding affinity
- Transmembrane domain that anchors the molecule to the T cell
- Intracellular costimulatory domain that enhances CAR-T cell persistence
- Intracellular signaling domain that initiates the biochemical cascade leading to immune activation
Costimulatory domains
CARs can have different costimulatory domains. Two such costimulatory domains are 4-1BB and CD28. See the table below for some characteristics of 4-1BB- and CD28-containing CARs.
