The first CAR-T cell therapy1
Novartis pioneered the introduction of CAR-T cell therapy as an approved treatment for B-cell malignancies. Since 2012, Novartis has partnered with the University of Pennsylvania, leading to the first approved CAR-T cell therapy in any disease state.1 This treatment is now approved in two indications across four continents—Asia, Australia, Europe, and North America.2-5 Novartis is committed to building on this innovation in CAR-T cell therapy and beyond, researching its CAR-T cell therapy in various clinical trials and constantly seeking new platforms to expand into new areas.6
Watch the video below to observe Novartis employees at the time when these clinical trials first began.
 VIDEO
VIDEO
This could be something remarkable for medicine and then remarkable for the world.
Vas Narasimhan, CEO of Novartis
The Novartis experience
Novartis has a heritage of developing CAR-T cell therapy, and has maintained leadership in the following areas as a result1:
Patient and caregiver support
Novartis established the industry-leading patient support platform for CAR-T cell therapy, providing resources to patients and caregivers for each step of the treatment process.
Safety and efficacy
The safety and efficacy profile shown in the global, phase 2 trials of CAR-T cell therapy help determine insurance coverage for its currently approved indications.8
Access
Novartis is committed to improving access, ranking #4 on the global Access to Medicine Index in 2022.2
Access to Medicine Index in 2022
Partnerships
Novartis is committed to maintaining and growing its diverse network of partnerships.2
Healthcare institutions:
Novartis has a history of building unique partnerships with healthcare institutions by managing CAR-T treatment centre onboarding and working closely with healthcare professionals on each patient case
Local health authorities:
Novartis has long experience partnering with local health authorities
Academic institutions:
Novartis is constantly seeking partnerships with academic institutions and their investigators to explore ways to improve outcomes and combine CAR-T cell therapies with other treatments
The future of CAR-T cell therapy
Research on CAR-T cell therapy focuses on improving efficacy and safety for patients, as well as exploring new cellular therapies and antigen targets to bring this potentially curative treatment to more patients.9 Explore the CAR-T cell graphic below to discover some key areas for research in both haematologic and solid tumour CAR-T cell therapy.10 Each area of research also includes the visualization of at least one example therapy within that category.
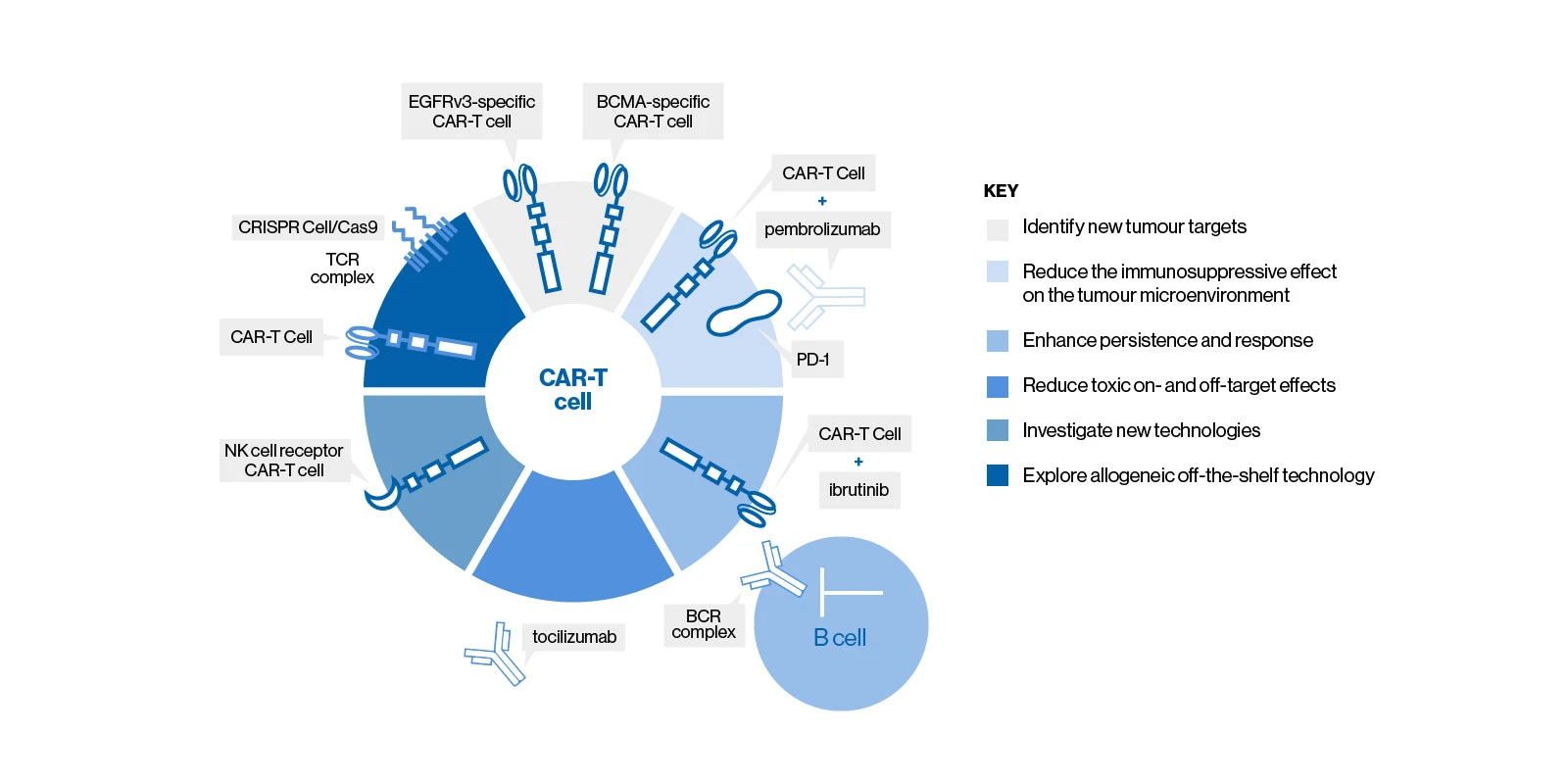
Identify new tumour targets9
Apart from better understanding the present and future of CD19-directed CAR-T cell therapy, work is also being done to engineer CAR-T cell therapies with various other target antigens
- Additional targets are being explored in haemotologic malignancies, including BCMA, CD22, and CD123
- Targets are also being explored in solid tumours, including EGFRv3, among others
Reduce the immunosuppressive effect on the tumour microenvironment10
Immune suppressive mechanisms in the microenvironment may help tumour cells evade the immune system
- The addition of a checkpoint inhibitor may help modulate immune evasion due to the microenvironment
Enhance persistence and response10
CAR-T cells must proliferate in the body and persist over time in some malignancies in order to have a potent, lasting effect
- Some biological tools for enhancing persistence and response may include dual receptor chimeric antigen receptors (CARs), new costimulatory domains, and B-cell receptor (BCR) signaling inhibitors
Reduce toxic on-target and off-target effects10
More control over CAR-T cell activity could help reduce off-target effects like cytokine release syndrome
- Tocilizumab administration can help manage cytokine release syndrome (CRS) severity postinfusion of CAR-T cell therapy9
Investigate new technologies9
Up-and-coming cellular therapies may improve the antitumour efficacy of CAR-T cell therapy
- These new technologies include natural killer (NK) cell receptors, gamma/delta T cells, and in vivo CAR-T cells9,11,12
Explore allogeneic off-the-shelf technology9,13
Off-the-shelf allo-CAR-T cell therapies do not require an individualized manufacturing process and therefore have the potential to reduce lead times
- Some gene editing or gene regulating tools under exploration include CRISPR/Cas9 and RNAi
We expect innovative medical approaches in areas such as gene therapy and immunology to hold the potential for the development of curative therapies.
Joerg Reinhardt, Chairman of the Board of Novartis
The Novartis cell and gene pipeline
Novartis is pioneering the future of CAR-T cell therapy and beyond, paving the way for innovation in cell and gene therapy. CD19-directed CAR-T cell therapies are currently commercially available for some B-cell malignancies.14 In addition to optimising treatment for CD19 markers, research is underway to develop CAR-T cell therapies that target new antigens.2,6
Novartis supports ongoing development program for registration and investigator-initiated trials for CAR-T cell therapy and beyond6:
CD19-directed CAR-T cell therapies are being studied in haematologic malignancies such as acute lymphoblastic leukaemia (ALL), diffuse large B-cell lymphoma (DLBCL), follicular lymphoma, chronic lymphocytic leukaemia (CLL), multiple myeloma, and acute myeloid leukaemia (AML)6
|
| Clinical trials are also underway to investigate CAR-T cell therapy in combination with immunotherapy and targeted therapies6 |
| Novartis is evaluating CAR-T cell therapy at different points in the DLBCL treatment paradigm that may be more tailored to individual patient needs, as well as in other lymphomas6 |
| Novartis is developing next-generation CAR-T cell therapies with the potential to target more than one antigen on the target cell surface in order to improve overall outcomes2 |
| Novartis is exploring new manufacturing technologies in an effort to continually improve upon this process2 |
To see what other cell and gene therapies Novartis is investigating outside of CAR-T cell therapy, please click here.
Always looking to expand its network of collaboration, Novartis seeks the opportunity to build new partnerships at all stages of development. To learn more about how to partner with Novartis, please click here. For more information about the Novartis pipeline, please click here.
