The global pandemic has left virtually no part of the world untouched, and moved our industry to collaborate like never before, and leverage our expertise and resources to support the global response to the COVID-19 pandemic. The RUXCOVID clinical trial is a testament to the Novartis commitment to contribute to these efforts.
The RUXCOVID clinical trial highlights the Novartis commitment to leverage our expertise and resources to support the global response to the COVID-19 pandemic.
While the RUXCOVID trial did not give us the results we hoped for, we will continue working with the medical community to analyze its findings, potentially improving understanding about COVID-19 and the role of JAK inhibition.
We are proud to be part of the international effort to address the global pandemic; and we would like to thank the front-line clinical teams and staff at each of the trial sites, as well as the patients who volunteered to participate on the trial and their loved ones.
Stages of COVID-19 and the RUXCOVID trial
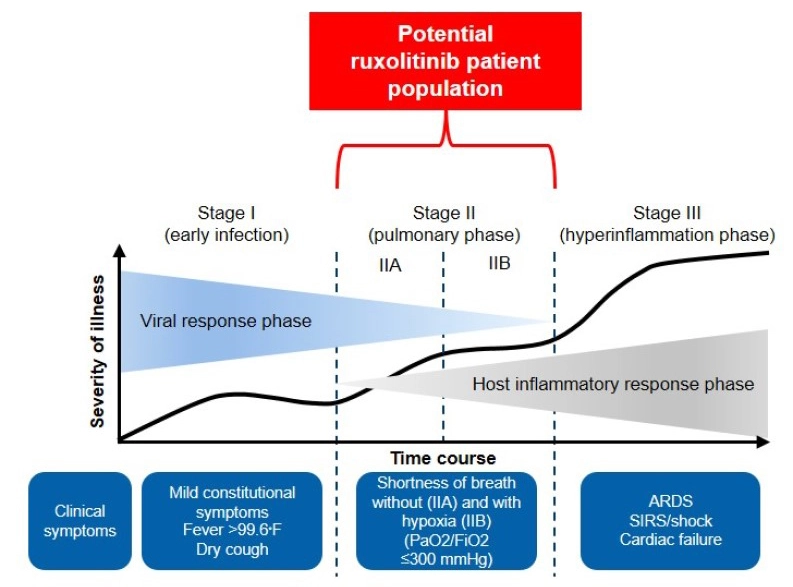
The RUXCOVID trial focused on hospitalized patients (aged ≥ 12 years) with COVID-19 infection, who were at risk for severe complications including ICU care, mechanical ventilation or death. This includes people whose lungs are affected and have low blood oxygen levels (Stage IIB).
Frequently asked questions
What is RUXCOVID?
RUXCOVID is a global, randomized, double-blind, placebo-controlled, 29-day, multi-center Phase III study evaluating the efficacy and safety of ruxolitinib plus standard of care (SoC) therapy in patients aged ≥12 years with COVID-19 associated cytokine storm.
More information is available at ClinicalTrials.gov.
Why was RUXCOVID initiated?
Ruxolitinib is a well-established JAK inhibitor and has been shown to reduce the levels of inflammatory cytokines in patients with myelofibrosis, secondary hemophagocytic lymphohistiocytosis (sHLH), and graft versus host disease (GvHD).
Patients with severe COVID-19 have substantially elevated cytokine levels leading to a state of hyperinflammation (cytokine release syndrome or CRS). Morbidity and mortality in severe COVID-19 is associated with this CRS. Ruxolitinib treatment results in the decrease of inflammation-related markers, including pro-inflammatory cytokines and activated T cells. Pre-clinical evidence as well as preliminary reports from independent studies suggest that ruxolitinib may reduce the number of patients with COVID-19 who require intensive care and mechanical ventilation.
Overall, these lines of evidence suggested that ruxolitinib could improve hyperinflammation associated with COVID-19 and have potential as a therapeutic option in patients with severe COVID-19 infection. Based on this, the randomized phase III trial, RUXCOVID, was initiated in order to further evaluate this in a prospective, randomized manner.
What was the outcome of the RUXCOVID study?
The Phase III study did not meet its primary endpoint. Initial data show there was no statistically significant reduction in the proportion of patients on ruxolitinib plus SoC therapy who experienced severe complications, including death, respiratory failure requiring mechanical ventilation or admission to the intensive care unit (ICU) by Day 29, compared to SoC alone. The trial also did not show clinically relevant benefit among secondary and exploratory endpoints including mortality rate by Day 29, and time to recovery (no longer infected, or ambulatory with no or minimal limitations).
Were there any safety concerns?
Ruxolitinib was generally well-tolerated; a comprehensive analysis including safety data is ongoing.
Does Novartis have other clinical trials in COVID-19?
Novartis is active in several key cross-industry research initiatives, the COVID-19 Therapeutics Accelerator, coordinated by the Bill & Melinda Gates Foundation, Wellcome, and Mastercard as well as a COVID-19 directed partnership supported by the Innovative Medicines Initiative (IMI). Novartis has also announced a collaboration with Molecular Partners to develop two DARPin® therapies designed for potential use against COVID-19, and the company is separately supporting COVID-19 related clinical investigations of several Novartis medicines.
Two medicines in early stage development are also being investigated focusing on stopping or slowing the body’s overactive immune response to COVID-19.
What else is Novartis doing to help combat COVID-19?
For the most up to date information on our ongoing response to the COVID-19 pandemic please take a look at novartis.com/coronavirus.
See our media release for more detailed information on the RUXCOVID trial.