Novartis believes all people with spinal muscular atrophy (SMA) – a rare, genetic, neuromuscular disease – should have access to gene therapy that addresses the root cause of their disease.
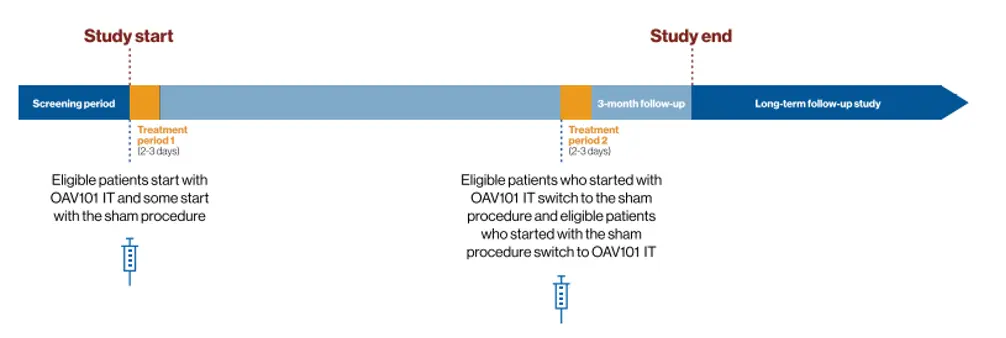
This is one reason why we’re conducting the STEER clinical trial, a global research study of the intrathecal (IT) administration of OAV101, an investigational gene therapy.
STEER is exploring the efficacy, safety, and tolerability of OAV101 IT in children and teens between 2 to <18 years of age who have SMA Type 2.1
All compounds under investigation are either investigational or being studied for (a) new use(s). Efficacy and safety have not been established for this new use. There is no guarantee that they will become commercially available for the use(s) under investigation.