
icon
-
Media Release
Novartis Kymriah® pivotal trial demonstrates strong response rates and a remarkable safety profile in relapsed or refractory follicular lymphoma
-
Media Release
Novartis Cosentyx receives FDA approval for treatment of children and adolescents with moderate to severe plaque psoriasis
-
Media Release
Novartis reports one year results of Phase III MERLIN study evaluating BEOVU® every four week dosing and provides update on BEOVU clinical program
-
Media Release
Novartis commits to addressing racial disparities in breast cancer screening, treatment and care
-
Story
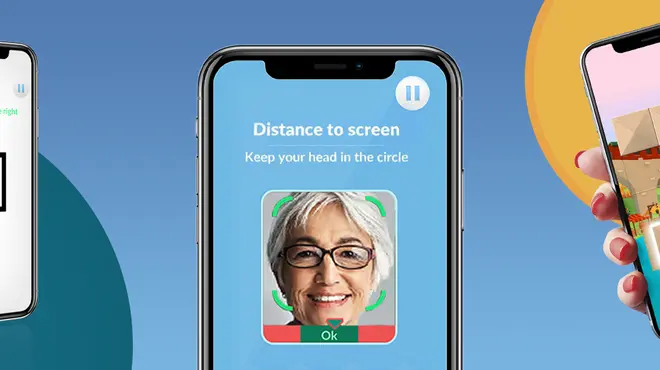 At-home app helps patients stay engaged and adherent to vision monitoring remotely
At-home app helps patients stay engaged and adherent to vision monitoring remotely -
Media Release
Novartis Phase III BEOVU® data show potential for fluid resolution in more diabetic macular edema patients with fewer injections versus aflibercept
-
Media Release
Novartis receives FDA approval of Xolair® (omalizumab) self-injection with prefilled syringe across all indications for appropriate patients
-
Media Release
Novartis Entresto® granted expanded indication in chronic heart failure by FDA
-
Story
 Telemedicine is helping patients in underserved communities access quality healthcare
Telemedicine is helping patients in underserved communities access quality healthcare -
Media Release
Novartis announces positive FDA Advisory Committee recommendation for use of Entresto® to treat patients with HFpEF
-
Media Release
Novartis Kisqali® demonstrates nearly five years median overall survival in metastatic breast cancer
-
Media Release
Novartis investigational STAMP inhibitor asciminib (ABL001) shows superior MMR rate to Bosulif®* in chronic myeloid leukemia trial
Pagination
- ‹ Previous page
- 1
- …
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- …
- 53
- › Next page